[:pb]ABNT NBR ISO 13485
A NCC é o primeiro OMD brasileiro acreditado para realização da certificação de SISTEMA DE GESTÃO DE PRODUTOS PARA SAÚDE com base na norma ABNT NBR ISO 13485.
A ISO 13485, derivada da internacionalmente reconhecida e aceita ISO 9001, especifica os requisitos do sistema de gestão da qualidade que pode ser usado por uma organização envolvida em um ou mais estágios do ciclo de vida de um produto para saúde, incluindo projeto e desenvolvimento, produção, armazenamento e distribuição, instalação, assistência técnica, disposição e descarte final.
A implementação e certificação da ISO 13485 para empresas que buscam entrar ou já atuam no setor de dispositivos médicos traz as seguintes vantagens:
- aumenta o acesso a mercados globais com a certificação;
- demonstra que você produz dispositivos médicos mais seguros e efetivos;
- melhoria nos processos internos e tomadas de decisão;
- criação de uma cultura de melhoria contínua;
- demonstra comprometimento com segurança e qualidade;
- demonstra capacidade de atender aos requisitos do cliente e legislativos.
Preparar-se para a certificação significa implementar um sistema de gestão de qualidade compatível com as leis e as regulamentações da indústria médica, além de comprovar sua capacidade de prover serviços e dispositivos médicos forma consistente aos requisitos regulamentares aplicáveis. A NCC é um organismo de certificação acreditado pela Cgcre (Coordenação Geral de Acreditação do Inmetro) e pode atender às suas necessidades de certificação.
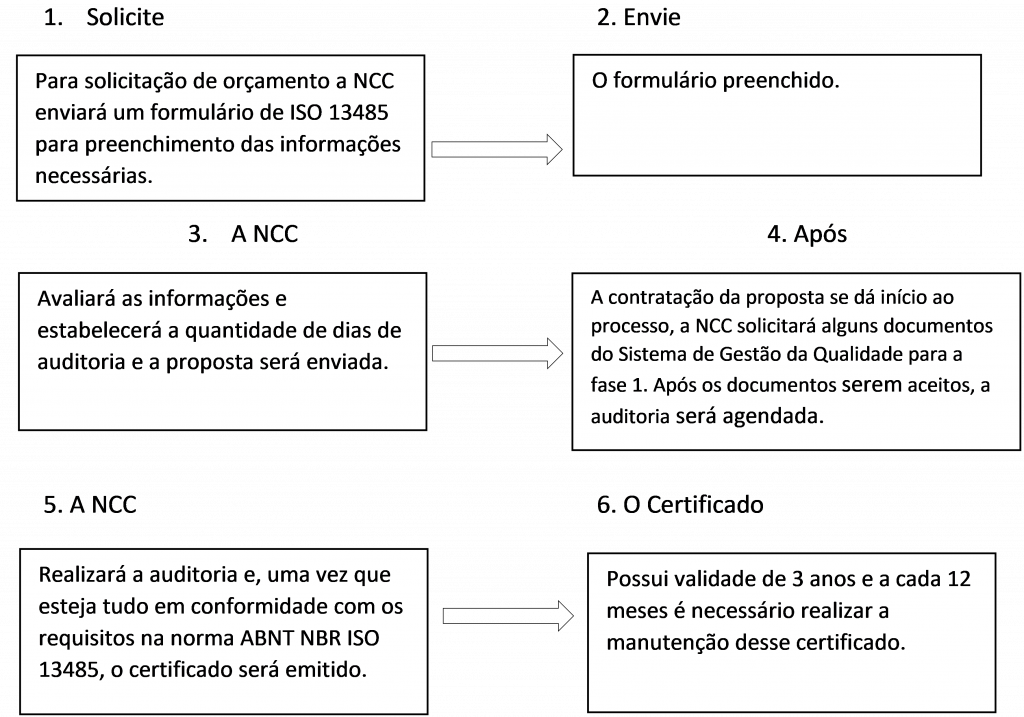
Como funciona o processo:
Para mais detalhes entre em contato com a NCC: Michele Moreira 19- 97826-9596.[:en]NCC is the first Brazilian OMD accredited to conduct the MANAGEMENT SYSTEM CERTIFICATION FOR HEALTH PRODUCTS based on the ABNT NBR ISO 13485 standard.
ISO 13485, derived from the internationally recognized and accepted ISO 9001, specifies the quality management system requirements that can be used by an organization involved in one or more stages of a medical device’s lifecycle, including design and development, production, storage, and distribution, installation, technical assistance, discarding, and final disposal.
The implementation and certification of ISO 13485 for companies seeking to enter or already operating in the medical device sector bring the following advantages:
- increases access to global markets with certification;
- demonstrates that you produce safer and more effective medical devices;
- improves internal processes and decision-making;
- creates a culture of continuous improvement;
- demonstrates a commitment to safety and quality;
- demonstrates the ability to meet customer and legislative requirements.
Preparing for certification means implementing a quality management system that complies with the medical industry’s laws and regulations and demonstrating your ability to provide medical devices and services consistently with applicable regulatory requirements. NCC is a certification body accredited by Cgcre (General Coordination for Accreditation of Inmetro) and can meet your certification needs.
How the process works:
Como funciona o processo:

For more details, contact NCC: Michele Moreira +55 19- 97826-9596.[:]
